 |
| CIRM graphic |
Randy Mills targeted the Food and Drug Administration (FDA), the agency that has life-or-death power over the introduction of stem cell therapies. "Give us our cures" was Mills' message for federal regulators.
The most recent forum for Mills was Fox News. The headline on an opinion piece that he co-authored with former Republican U.S. Sen. Bill Frist, said,
"Cell therapy reversed blindness for 47,000 patients in 2015. So why is it against the law?"
And in a presentation earlier this month to the Bipartisan Policy Center in Washington, D.C., Mills said that the current FDA "paradigm" for new stem cell therapies has been inconsistent, selective and chilling.
"By having a system that approves nothing after 15 years, we are neither protecting nor helping those in need."Mills said that FDA regulations should "be scaled to more accurately reflect the risks, be balanced against the very real consequences of doing nothing and be fairly and consistently applied."
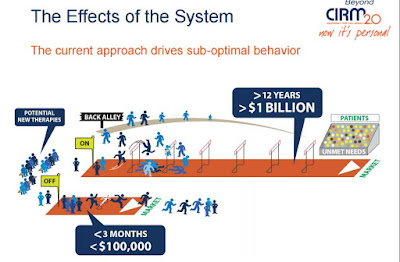
The campaign by the California Institute for Regenerative Medicine (CIRM), as the stem cell agency is formally known, grew out of work last year on its strategic plan. A survey of stakeholders showed that 70 percent of them identified the FDA as an impediment to development of commercial therapies.
Kevin McCormack, senior director of communications for CIRM, wrote about Mills' piece on Fox on the agency's blog, The Stem Cellar. McCormack said,
"A lot of people are frustrated with the US Food and Drug Administration (FDA) and its woefully slow process for approving stem cell therapies. That’s one of the reasons why we started the CIRM Stem Cell Champions campaign, to gather as many like-minded supporters of stem cell research as possible and help to change the way the FDA works, to create a more efficient approval process."In his presentation in Washington, Mills was careful to point out that CIRM is not opposed to the FDA or its regulation. He said CIRM just wants better regulation that will help bring therapies to patients by speeding their approval and balancing risk.
In response to a question about how Fox News happened to carry the Mills' piece, McCormack, replied,
"It was something Randy and Senator Frist had been talking about doing for a while, and why not Fox News, it has a big audience."Other big audiences for CIRM's message will be in the San Francisco Bay area next month: BIO, the annual biotech industry convention, which is expected to draw more than 15,000 from June 6 to June 9, and the annual meeting June 22-25 of the International Society for Stem Cell Research, which expects to see more than 4,000 attendees. CIRM is expected to have a strong presence, relatively speaking, at both events.
 |
| The stem cell agency says FDA practices lead to selective enforcement of its regulations. CIRM graphic |
“The FDA is inhibitory to translation of stem cell therapies to patients. There is no sense in regulating MSCs as a drug.”
ReplyDeleteProfessor Arnold Caplan of Case Western Reserve University, widely regarded as "The Father of the Mesenchymal Stem Cell".
Interview with Arnold Caplan, Part 4: the FDA and the Future Knoepfler Lab Stem Cell Blog, April 7, 2013
We now have the chance to rectify this situation with legislation aimed at accelerating approval of cellular therapies. Please review the information on The Regrow Act, currently before Congress, and use the email form to contact your reps.
ReplyDeleteSend email to your congressional reps here http://www.celltherapynow.org/
ReplyDeleteRevision of regulations should take place but patient safety is paramount. The REGROW Act in it's current form does not respect this and would endanger many patients exposed to drugs with only preliminary safety. This has been recognized by multiple patient advocacy groups, who now oppose REGROW http://www.michaeljfox.org/foundation/news-detail.php?mjff-signs-letter-opposing-the-regrow-act
ReplyDelete