The $3 billion California stem cell agency reports that its governing board will meet 12 times this year, mostly in teleconference sessions that will also have access on the Internet.
The business of the board is largely ratifying awards approved earlier by its grant reviewers. However, its financial survival will be a topic of regular discussion this year. The agency expects to run out of cash for new awards before the end of the year. It is hoping that voters will renew its funding with $5 billion in the fall of 2020.
Four "in-person" meetings are scheduled this year during which most of the board will gather at the agency's headquarters in Oakland. They are scheduled to occur in March, May, September and December. Those will be also accessible on the Internet, both for listening or participating.
The meetings of the board offer opportunities for members of the public, including researchers and business representatives, to speak directly to board members and staff of the agency. The in-person sessions are particularly valuable in that regard because of the informality before and after the meetings, as well as during breaks. Seating is somewhat limited at the meetings at the headquarters of the agency.
Dates and times of the meetings may change, however, and should be double-checked as the sessions near. The first meeting of the year is scheduled to be conducted via teleconferencing and is set for Jan. 30.
The official name of the board is the Independent Citizens' Oversight Committee. The formal name of the stem cell agency is the California Institute for Regenerative Medicine.
With more than 3.0 million page views and more than 5,000 items, this blog provides news and commentary on public policy, business and economic issues related to the $3 billion California stem cell agency. David Jensen, a retired California newsman, has published this blog since January 2005. His email address is djensen@californiastemcellreport.com.
Monday, January 07, 2019
Thursday, January 03, 2019
Hype and Fraud and Their Impact on the California Stem Cell Program
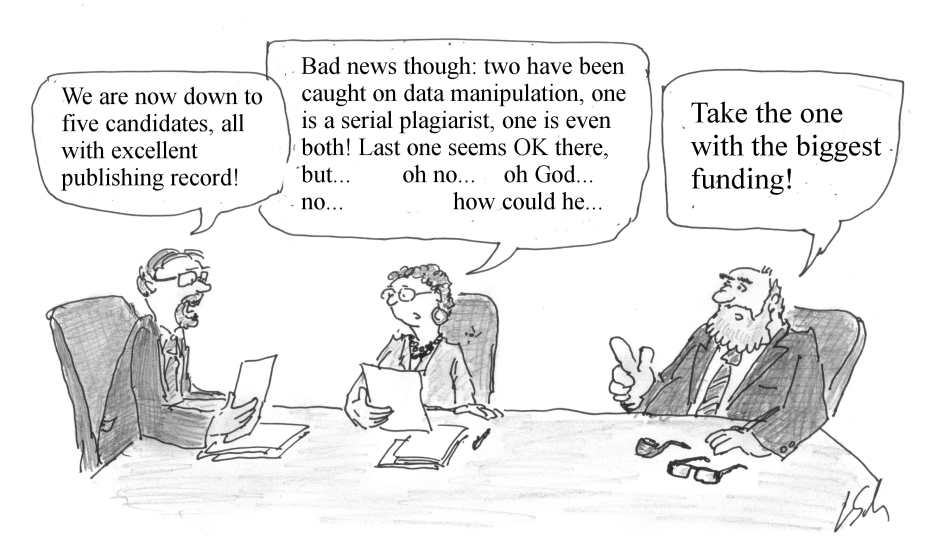 |
| Cartoon by Johan Thyberg from "For Better Science" |
The questions it raises have particular relevance in the stem cell field, which is still very much in a formative stage.
Aaron Carroll, a professor of pediatrics at the University of Indiana, produced the opinion piece for the Times. He wrote,
"How might grant funding and career advancement — even the potential for fame — be biasing researchers? How might the desire to protect reputations affect the willingness to accept new information that reverses prior findings?"
He also said,
"Moves toward open science, and for a change in the academic environment that currently incentivizes secrecy and the hoarding of data, are perhaps our best chance to improve research reproducibility Recent studies have found that an alarmingly high share of experiments that have been rerun have not produced results in line with the original research."
Questions arise with some regularity about hype and fraud in stem cell research. Back in 2014, bioethicist Art Caplan asked, “Why so Much Fake, Unduplicable Stem Cell Research?”
Just this past fall, a multimillion-dollar stem cell research scandal involving Harvard surfaced once again in the news.
Credibility, of course, is everything in science. For the California stem cell agency, it is a matter of survival. It will be out of cash for new awards in less than 12 months and is hoping voters will then give it an additional $5 billion. More news about stem cell hanky panky, wherever it occurs, will not serve the agency well when it makes its pitch once again to the people of the Golden State.
Wednesday, January 02, 2019
Top Ten Blog Hits by the $3 Billion California Stem Cell Agency
The California stem cell agency produces a blog called "The Stem Cellar," which regularly features fine pieces on stem cell matters in general as well as articles about its own activities.
This week the agency produced a list of the most popular postings on its site. Here is the rundown on the top 10.
This week the agency produced a list of the most popular postings on its site. Here is the rundown on the top 10.
- "New stem cell treatment for ALS may slow disease progression
- "Can stem cell therapies help ALS patients
- "Stem cell study holds out promise for kidney disease
- "Your guide to awesome stem cell conferences in 2018
- "Young man with spinal cord injury regains use of hands and arms after stem cell therapy
- "jCyte shares encouraging news update about clinical trial for Retinitis Pigmentosa
- "Stem cell patch restores vision in patients with age-related macular degeneration
- "The 10 most popular stem cell stories of 2017 (is that cheating to include a story about the most popular stories of 2017 in a blog about the most popular blogs of 2018?)
- "Could stem cells help beat multiple sclerosis
- "One-time lasting treatment for Sickle Cell Disease may be on the horizon according to new CIRM-funded study"
Sunday, December 23, 2018
Serious Infections and Fecal Contamination Alleged; Feds Battle Rising Tide of Dubious Stem Cell Clinics
The federal government last week stepped up its efforts to curb dubious stem cell clinics, declaring that 12 persons have been hospitalized for infections as the result of treatments involving a San Diego firm.
Some of the products from the firm have been found to have been contaminated with fecal bacteria .
The federal action (see here and here) comes after years of ignoring the problem, both by the Food and Drug Administration and state regulators. Meanwhile, the growth of unregulated clinics as multiplied. Current estimates are that as many as 700 or more clinics exist in the United States, compared to at least 570 in 2016.
California has the biggest share of the clinics, which is not unusual since it is the most populous state in the nation. Legislation backed by the California stem cell agency is expected to be introduced in California next month to step up regulation of dubious clinics.
The federal enforcement action came in the form of a "warning" letter from the FDA and involved a firm called Genetech (no relation to the well-respected biotech firm Genentech, but obviously a name designed to lure unsuspecting patients.)
The FDA said 12 patients have contracted serious infections as the result of injections involving Genetch procedures and products. The Center for Disease Control has reported that some of its unopened products contain E. coli and E. faecalis.
Genetech has not responded to multiple efforts by media to obtain a comment on the federal action.
Some stem cell scientists, most prominently UC Davis researcher Paul Knoepfler, have warned repeatedly for years about the unregulated treatments. In addition to the harm to patients, they have noted that the activities of the dubious clinics damage the reputation of the field in general. (See here, and here and here.
Some of the products from the firm have been found to have been contaminated with fecal bacteria .
The federal action (see here and here) comes after years of ignoring the problem, both by the Food and Drug Administration and state regulators. Meanwhile, the growth of unregulated clinics as multiplied. Current estimates are that as many as 700 or more clinics exist in the United States, compared to at least 570 in 2016.
California has the biggest share of the clinics, which is not unusual since it is the most populous state in the nation. Legislation backed by the California stem cell agency is expected to be introduced in California next month to step up regulation of dubious clinics.
The federal enforcement action came in the form of a "warning" letter from the FDA and involved a firm called Genetech (no relation to the well-respected biotech firm Genentech, but obviously a name designed to lure unsuspecting patients.)
The FDA said 12 patients have contracted serious infections as the result of injections involving Genetch procedures and products. The Center for Disease Control has reported that some of its unopened products contain E. coli and E. faecalis.
Genetech has not responded to multiple efforts by media to obtain a comment on the federal action.
Some stem cell scientists, most prominently UC Davis researcher Paul Knoepfler, have warned repeatedly for years about the unregulated treatments. In addition to the harm to patients, they have noted that the activities of the dubious clinics damage the reputation of the field in general. (See here, and here and here.
Monday, December 17, 2018
A Deep Probe by STAT into the Man Behind the CRISPR Babies
Want to know more about the researcher who spawned what are now known as the CRISPR babies?
STAT published a cracker jack of a piece this morning about He Jiankui, including details about his life and his pathway into the gene editing game.
STAT promoted the article this morning in a newsletter with this squib:
"Every superhero, and antagonist, has his own origin story. He Jiankui, the scientist who stunned the world with a claim that he had already gene-edited two baby girls, has one too."
The lengthy article was written by Sharon Begley and Andrew Joseph and covers He's path from physics to biology. They quoted UC Berkeley scientist Jennifer Doudna as saying,
“His demeanor was an odd combination of hubris and naivete. He was very confident in his work, and not totally understanding what an explosion he had caused.The STAT piece is based on extensive reporting. Its authors wrote,
"With details reported for the first time, it describes the many times He met with and spoke before some of the world’s leading genome-editing experts, the low opinion they had of his research, and the hints he dropped about his grandiose aspirations. It is based on interviews in Hong Kong and with experts on four continents, with scientists and others who have crossed paths with He, as well as on documents and published accounts. He did not reply to requests for an interview."The article's final paragraph ends like this:
"Even those who condemn his experiment doubt it will be more than a speed bump on the road to editing of embryos to prevent severe inherited diseases. 'We have to acknowledge there is interest in using [CRISPR] clinically,' Doudna said. To those calling for a moratorium or an outright ban on such research, she has one response: 'It’s too late.'"
Thursday, December 13, 2018
California Stem Cell Agency Scores 50 Clinical Trials, Says It "Just Getting Started"
OAKLAND, Ca. -- California's ambitious stem cell agency this morning chalked up involvement in 50 clinical trials, an achievement that was regarded by some as unattainable 10 years ago.
The watershed event came when directors of the agency approved a $6.2 million proposal to help fight lymphoma.
Following the action, the first person ever to participate in a CIRM human embryonic stem cell trial, Richard Lajara, praised the efforts of the agency, which is running out of cash.
He said the people of California have a responsibility to move forward with stem cell research and to continue to fund the agency. Lajara's appeal followed a statement from the agency that 35 persons are alive today because of clinical trials that it has helped to finance.
Lajara, who is paralyzed from the waist down, was enrolled in 2011 in the spinal injury trial initiated by Geron, Inc., and backed by the agency.
Jonathan Thomas, chairman of the agency, which is formally called the California Institute for Regenerative Medicine(CIRM), said in a statement,
The cost of trials varies widely but can run into hundreds of millions of dollars, which could rapidly devour the $3 billion the agency had to spend, the reasoning went. Clinical trials are the last stage before a treatment is approved for widespread use by the federal government.
CIRM's first strategic plan in December 2006, which covered a 10 year period, anticipated some involvement in phase one and two trials. But it said,
Today, the agency has committed $541 million to clinical trials, including four that are in phase three, the ultimate trial hurdle to clear before a treatment wins approval. The phase three trials involve kidney disease and ALS.
The 14-year-old agency is now down to its last $144 million. Money for new awards will run out at the end of next year. CIRM is pinning its hopes for continued life on a proposed $5 billion ballot measure on the November 2020 ballot.
Key to winning approval would be a favorable result from its clinical trials -- one that would resonate with the voters. Their expectations were raised in 2004 by the ballot campaign that led to creation of the agency. However, the agency has yet to produce a stem cell treatment that is widely available.
The watershed event came when directors of the agency approved a $6.2 million proposal to help fight lymphoma.
Following the action, the first person ever to participate in a CIRM human embryonic stem cell trial, Richard Lajara, praised the efforts of the agency, which is running out of cash.
He said the people of California have a responsibility to move forward with stem cell research and to continue to fund the agency. Lajara's appeal followed a statement from the agency that 35 persons are alive today because of clinical trials that it has helped to finance.
Lajara, who is paralyzed from the waist down, was enrolled in 2011 in the spinal injury trial initiated by Geron, Inc., and backed by the agency.
Jonathan Thomas, chairman of the agency, which is formally called the California Institute for Regenerative Medicine(CIRM), said in a statement,
"We have come a long way in the past seven and a half years, helping advance the field from its early days to a much more mature space today, one capable of producing new treatments and even cures.
"But we feel that in many ways we are just getting started, and we intend funding as many additional clinical trials as we can for as long as we can."In the early days of the agency, Robert Klein, its first chairman, and others widely regarded major participation in clinical trials, especially phase three, as largely beyond the financial capability of the agency. The cost was prohibitive, they said.
The cost of trials varies widely but can run into hundreds of millions of dollars, which could rapidly devour the $3 billion the agency had to spend, the reasoning went. Clinical trials are the last stage before a treatment is approved for widespread use by the federal government.
CIRM's first strategic plan in December 2006, which covered a 10 year period, anticipated some involvement in phase one and two trials. But it said,
"Because of their expense and because of the time required to reach this stage of clinical development, CIRM is unlikely to fund Phase III trials over the time span of the strategic plan."
The first patient to enroll in a CIRM clinical trial did not come until late 2011.
Today, the agency has committed $541 million to clinical trials, including four that are in phase three, the ultimate trial hurdle to clear before a treatment wins approval. The phase three trials involve kidney disease and ALS.
The 14-year-old agency is now down to its last $144 million. Money for new awards will run out at the end of next year. CIRM is pinning its hopes for continued life on a proposed $5 billion ballot measure on the November 2020 ballot.
Key to winning approval would be a favorable result from its clinical trials -- one that would resonate with the voters. Their expectations were raised in 2004 by the ballot campaign that led to creation of the agency. However, the agency has yet to produce a stem cell treatment that is widely available.
Wednesday, December 12, 2018
Trump, Fetal Tissue and the California Stem Cell Agency
The California stem cell agency says the Trump Administration moves against research involving fetal tissue have had no impact on the projects that it is financing, at least so far.
The agency, formally known as the California Institute for Regenerative Medicine (CIRM), was responding to a question originally raised by a reader of the California Stem Cell Report.
The inquiry was triggered by a number of reports over the last few months concerning the federal direction away from fetal tissue, a move that one researcher, Warner Greene of the Gladstone Institutes in California, called "scientific censorship of the worst kind."
The California Stem Cell Report queried CIRM yesterday about the federal actions, asking whether they have had "any impact, direct or indirect, on CIRM awards, existing or likely in the future."
The agency, formally known as the California Institute for Regenerative Medicine (CIRM), was responding to a question originally raised by a reader of the California Stem Cell Report.
The inquiry was triggered by a number of reports over the last few months concerning the federal direction away from fetal tissue, a move that one researcher, Warner Greene of the Gladstone Institutes in California, called "scientific censorship of the worst kind."
The California Stem Cell Report queried CIRM yesterday about the federal actions, asking whether they have had "any impact, direct or indirect, on CIRM awards, existing or likely in the future."
Kevin McCormack, senior director for CIRM communications, replied, noting that the federal move is relatively recent.
"It could mean an increase in applications that use fetal tissue but it’s too soon to tell. Regardless, this is why the people of California created CIRM, so we don’t have to worry about federal funding for potentially life-saving research. Because we are independent, we can fund what we think is the best science."McCormack alluded to the ballot initiative in 2004 that established the agency. The campaign was largely based on the need to bypass the Bush administration's restrictions on stem cell research. The anti-fetal tissue effort is likely to be the first step towards resurrecting similar restrictions on stem cell research.
Politically and ironically speaking, new federal restrictions on human embryonic stem cell research could build support for continued funding of CIRM, which is hoping for passage of a proposed $5 billion bond measure on the November 2020 ballot.
While fresh restrictions are not good for the field overall, their imposition could help to preserve the stem cell agency. Any ballot campaign needs a nasty villain to campaign against.
And without Bush to campaign against in 2004, the stem cell agency probably would never have come into existence.
Here are links to a few of the recent stories on the Trump fetal tissue move: Washington Post, Science, STAT.
Tuesday, December 11, 2018
Walking a Thin Line at the California Stem Cell Agency
California's stem cell agency has a delicate dance to perform as it edges closer to seeing a $5 billion measure on the ballot to provide more funding for its efforts.
The dance involves a prohibition on state agencies spending public money on behalf of bond measures. The $3 billion agency has a legitimate responsibility to keep the public informed about its activities, but when does that task step over a legal line?
CALmatters, an online news site devoted to state government matters, touched on the issue this morning. Dan Morain, the site's senior editor, wrote about a couple of cases in the last election.
In his morning newsletter "What Matters," he cited a tiny fine imposed on the Bay Area Rapid Transit District and another case involving an $800,000 expenditure by Los Angeles County board of supervisors, which is unresolved.
As for the stem cell agency, it is certain to be accused of using public money to support any ballot measure that may emerge in 2020.
That would be part of the political tactics of opponents to the measure -- a portrayal of the stem cell agency as unworthy of voter trust.
The agency is treading lightly in this area right now, a position that will serve it well as more clarity emerges on whether a ballot initiative will actually surface on the November 2020 ballot.
Sunday, December 09, 2018
California Setting Stage for Crackdown on Dubious Stem Cell Clinics
The California stem cell agency, state regulators and lawmakers are taking aim at the more than 100 dubious, unregulated "stem cell" clinics now operating in the Golden State.
The goal is to curb clinics that are using what they describe as stem cells in treatments costing thousands of dollars but that have not been tested scientifically. Lawsuits have been filed around the country alleging damage to patients that includes blindness.
Art Torres, vice chairman of the state stem cell agency, is now working with lawmakers to formulate legislation that is expected to be introduced by the end of January.
At the same time, the State Medical Board, which licenses and regulates physicians, has chartered a task force to look into the the growing business.
Earlier this fall, Torres told the governing board of
the California Institute for Regenerative Medicine (CIRM), as the agency is formally known, that he was engaged with Assemblyman Kevin Mullin, D-San Mateo, on a bill.
Torres, a former state lawmaker, said the legislation is expected to involve certification of clinics by a state department. He said,
At the same time, the State Medical Board, which licenses and regulates physicians, has chartered a task force to look into the the growing business.
Earlier this fall, Torres told the governing board of
 |
| Kevin Mullin, LA Times photo |
Torres, a former state lawmaker, said the legislation is expected to involve certification of clinics by a state department. He said,
"It involves a number of issues which we (CIRM) really can't be involved with in terms of licensing, but we certainly can be involved with the parameters and the distinctions that we ought to raise as to what constitutes an appropriate stem cell clinic in California."
The Medical Board is scrutinizing the promotional practices and harm caused by the clinics with the intent of crafting regulations to curb abuses.
"There is reasonable concern about a growing number of providers and clinics in the United States that are undermining the field. Such providers and clinics have been known to apply, prescribe or recommend therapies inappropriately, over-promise without sufficient data to support claims, and exploit patients who are often in desperate circumstances and willing to try any proposed therapy as a last resort, even if there is excessive cost or scant evidence of efficacy."
Paul Knoepfler, a UC Davis stem cell scientist who has long been involved in examination of dubious clinics, has reported that at least 100 such clinics exist in California.
Writing on his blog Nov. 30, Knoepfler said,
"Broadly, it may be going rapidly from the best of times to the worst of times for unproven stem cell clinics in the U.S., which would be a very good thing for patients and the stem cell field, if it actually happens. We’ll see."
Labels:
fraud,
stem cell clinics,
unregulated treatments
Friday, December 07, 2018
Former Biotech Maven Steve Burrill Sentenced to Prison
Famed and legendary he was called. Now he is facing 2.5 years in prison.
He is Steve Burrill, who once was a featured life science maven/financier/visionary at international conferences such as BIO, which attracts upwards of 16,000 persons annually. Burrill also conducted a well-attended stem cell conference in San Francisco that showcased California's stem cell agency shortly after it came into existence.
Burrill, who was based out of San Francisco, was sentenced this week for defrauding investors and falsifying his tax returns. He pled guilty to siphoning off $18 million from his companies.
Here are links to the news stories on the case: GEN News, STAT, Xconomy.
 |
| Steve Burrill, wn.com photo |
Burrill, who was based out of San Francisco, was sentenced this week for defrauding investors and falsifying his tax returns. He pled guilty to siphoning off $18 million from his companies.
Here are links to the news stories on the case: GEN News, STAT, Xconomy.
Thursday, December 06, 2018
California Stem Cell Agency Slated to Award $6.2 Million to Fight Lymphoma
California's $3 billion stem cell agency, which turned 14 last month, is expected next Thursday to give away another $6.2 million as it continues its efforts to fulfill the expectations of the voters who created it in 2004.
Also possibly on tap is an update on the status of efforts to raise privately some $200 million to tide over the agency as it looks forward to 2020 and a possible ballot measure to provide it with another $5 billion.
Known formally as the California Institute for Regenerative Medicine (CIRM), the agency expects to run out of cash for new awards in about 12 months. Its award budget for 2019 now stands at $144 million. Presumably, another $28.4 million can be added, which is the uncommitted cash for research left over from this year.
The agency subsists on state bond funds approved by voters. Its bond issuance authority, however, is expiring. No other source of funding was provided by voters, and the agency has no expectations of being financed on annual basis by the legislature.
The 2004 ballot campaign that created the first-ever such agency in California history raised high hopes a stem cell therapy was right around the corner. CIRM has not yet backed a treatment that is available for widespread use. However, it is helping to fund 49 clinical trials, the last steps before a treatment is approved for the marketplace.
The application (CLIN2-11371) before the board next week has already been approved by the agency's reviewers. Normal practice is for the board to ratify in public earlier decisions made in private by reviewers.
The name of the recipient has been withheld by the agency until after ratification, as is the agency's standard practice. The proposal seeks to continue a phase one clinical trial to help treat lymphoma.
The CIRM summary of the review of the application said the goal of the research is to "ameliorate or accelerate recovery from toxicities related to high-dose chemotherapy followed by HDT-ASCT for the treatment of lymphoma and other cancers."
CIRM said the method would involve "genetically engineered CD31+ cells derived from human umbilical vein tissue (engineered HUVEC)."
CIRM Chaiman Jonathan Thomas has been working to raise the private funds to help support the agency beyond next year. He often reports on his progress at board meetings.
The CIRM board meeting will be based in Oakland. Offsite locations where the public can participate are located in Stanford and San Francisco. The public can also log in online and ask questions or make comments. Instructions on how to participate are contained on the meeting agenda. If you are not familiar with the procedure, it is useful to log in about 10 minutes prior to the meeting's start (10 a.m. PST) to avoid technical difficulties.
Also possibly on tap is an update on the status of efforts to raise privately some $200 million to tide over the agency as it looks forward to 2020 and a possible ballot measure to provide it with another $5 billion.
Known formally as the California Institute for Regenerative Medicine (CIRM), the agency expects to run out of cash for new awards in about 12 months. Its award budget for 2019 now stands at $144 million. Presumably, another $28.4 million can be added, which is the uncommitted cash for research left over from this year.
The agency subsists on state bond funds approved by voters. Its bond issuance authority, however, is expiring. No other source of funding was provided by voters, and the agency has no expectations of being financed on annual basis by the legislature.
The 2004 ballot campaign that created the first-ever such agency in California history raised high hopes a stem cell therapy was right around the corner. CIRM has not yet backed a treatment that is available for widespread use. However, it is helping to fund 49 clinical trials, the last steps before a treatment is approved for the marketplace.
The application (CLIN2-11371) before the board next week has already been approved by the agency's reviewers. Normal practice is for the board to ratify in public earlier decisions made in private by reviewers.
The name of the recipient has been withheld by the agency until after ratification, as is the agency's standard practice. The proposal seeks to continue a phase one clinical trial to help treat lymphoma.
The CIRM summary of the review of the application said the goal of the research is to "ameliorate or accelerate recovery from toxicities related to high-dose chemotherapy followed by HDT-ASCT for the treatment of lymphoma and other cancers."
CIRM said the method would involve "genetically engineered CD31+ cells derived from human umbilical vein tissue (engineered HUVEC)."
"There are currently only a few moderately effective treatments available to reduce the toxic side effects associated with aggressive cancer treatments – hence a high unmet medical need. New approaches are urgently needed to both improve quality of life and reduce the risks of high dose therapy."The summary said that the $6.2 million award would be backed by $2.7 million from the recipient.
CIRM Chaiman Jonathan Thomas has been working to raise the private funds to help support the agency beyond next year. He often reports on his progress at board meetings.
The CIRM board meeting will be based in Oakland. Offsite locations where the public can participate are located in Stanford and San Francisco. The public can also log in online and ask questions or make comments. Instructions on how to participate are contained on the meeting agenda. If you are not familiar with the procedure, it is useful to log in about 10 minutes prior to the meeting's start (10 a.m. PST) to avoid technical difficulties.
Labels:
cancer,
cirm awards,
clinical trials,
lymphoma
Friday, November 30, 2018
The Valley of Death and the California Stem Cell Agency: Luring Deep Pocket Investors
The California stem cell agency this week is tooting a $150 million horn and heralding its efforts to assist stem cell businesses with development of therapies that could ease the travails of everything from cancer to blindness.
It is all about a financial "valley of death" that can imperil biotech firms as they seek to turn research into an actual product that can be used by patients. The latest poster child for the California Institute for Regenerative Medicine (CIRM), as the agency is formally known, is a San Diego firm called ViaCyte.
The enterprise has received more cash -- $72 million -- from CIRM than any other business. CIRM is facing its own valley of death next year, when its taxpayer cash will run out.
Writing yesterday on the CIRM blog, the agency's communications director, Kevin McCormack, said,
Nonetheless it is an important part of the CIRM story, one that will be tested perhaps in November 2020. That's when the $3 billion agency hopes to see a measure on the ballot that will give it another $5 billion. So far the agency, created in 2004 by a ballot initiative, has not fulfilled voter expectations that it would produce a stem cell therapy that is widely available. And it will need a good yarn to inspire voters once again in 2020.
It is all about a financial "valley of death" that can imperil biotech firms as they seek to turn research into an actual product that can be used by patients. The latest poster child for the California Institute for Regenerative Medicine (CIRM), as the agency is formally known, is a San Diego firm called ViaCyte.
The enterprise has received more cash -- $72 million -- from CIRM than any other business. CIRM is facing its own valley of death next year, when its taxpayer cash will run out.
Writing yesterday on the CIRM blog, the agency's communications director, Kevin McCormack, said,
"CIRM was created, in part, to help...great ideas get through the valley (of death). That’s why it is so gratifying to hear the news today from ViaCyte – that is developing a promising approach to treating type 1 diabetes – that they have secured $80 million in additional financing.
"The money comes from Bain Capital Life Sciences, TPG and RA Capital Management and several other investors. It’s important because it is a kind of vote of confidence in ViaCyte, suggesting these deep-pocket investors believe the company’s approach has real potential."McCormack continued,
"CIRM has been a big supporter of ViaCyte for several years, investing more than $70 million to help them develop a cell therapy that can be implanted under the skin that is capable of delivering insulin to people with type 1 diabetes when needed. The fact that these investors are now stepping up to help it progress suggests we are not alone in thinking this project has tremendous promise.
"But ViaCyte is far from the only company that has benefitted from CIRM’s early and consistent support. This year alone CIRM-funded companies have raised more than $1.0 billion in funding from outside investors; a clear sign of validation not just for the companies and their therapies, but also for CIRM and its judgment.
"This includes:One could argue that these companies could have found backing from other sources than the stem cell agency. One could argue that state government should not be in a business that is too risky for even the vaunted world of venture capitalists.
- Humacyte raising $225 million for its program to help people battling kidney failure
- Forty Seven Inc. raising $113 million from an Initial Public Offering for its programs targeting different forms of cancer
- Nohla Therapeutics raising $56 million for its program treating acute myeloid leukemia"
Nonetheless it is an important part of the CIRM story, one that will be tested perhaps in November 2020. That's when the $3 billion agency hopes to see a measure on the ballot that will give it another $5 billion. So far the agency, created in 2004 by a ballot initiative, has not fulfilled voter expectations that it would produce a stem cell therapy that is widely available. And it will need a good yarn to inspire voters once again in 2020.
Labels:
biotech financing,
bond election,
CIRM PR,
stem cell business
Wednesday, November 28, 2018
The Genetically Altered Babies Story: More Information Surfaces on Researcher's California Links
More details are emerging this week concerning the California connections of the man behind what are being described as the world's first gene-edited babies.
The scientist is He Jiankui, who spent two years in a lab at Stanford University, according to the lab's web site.
Lisa Krieger of the San Jose Mercury News has produced a roundup of the information about the researcher's activities in the Golden State. They include the connections with Stephen Quake of Stanford, who heads the lab where He Jiankui worked from 2010 to 2012.
She reported that Quake is declining any comment on He Jiankui.
Also mentioned in Krieger's piece are Mark Dewitt of UC Berkeley, William Hurlburt of Stanford and Jennifer Doudna, also of Berkeley.
Another useful piece exploring He Jiankui's training was produced by Sharon Begley, Andrew Joseph and Rebecca Robbins at STAT. The article takes a broad look at the researcher's training and background.
One cautionary note: The "facts" in this ongoing tale sometimes seem in conflict and sometimes murky. UC Davis' Paul Knoepfler raised the matter on his blog yesterday in an item headlined,
Lisa Krieger of the San Jose Mercury News has produced a roundup of the information about the researcher's activities in the Golden State. They include the connections with Stephen Quake of Stanford, who heads the lab where He Jiankui worked from 2010 to 2012.
She reported that Quake is declining any comment on He Jiankui.
Also mentioned in Krieger's piece are Mark Dewitt of UC Berkeley, William Hurlburt of Stanford and Jennifer Doudna, also of Berkeley.
Another useful piece exploring He Jiankui's training was produced by Sharon Begley, Andrew Joseph and Rebecca Robbins at STAT. The article takes a broad look at the researcher's training and background.
One cautionary note: The "facts" in this ongoing tale sometimes seem in conflict and sometimes murky. UC Davis' Paul Knoepfler raised the matter on his blog yesterday in an item headlined,
"Trying to connect the dots on CRISPR baby story paints a dark, cloudy picture."
Even determining the number of years He Jiankui worked at Stanford is in question. Quake's lab clearly reports two years. STAT reports that it was "about a year" without identifying a source. A relatively minor point, but if that can't be nailed down, what else is missing? As Knoepfler wrote, much murkiness exists in the morass of stories and commentary that has emerged this week.
The caveat for those who follow this matter? As the old adage goes,
The caveat for those who follow this matter? As the old adage goes,
"Even if your mother says it's true, check it out."
Labels:
bioethics,
crispr,
gene editing,
genetic alteration
Tuesday, November 27, 2018
The California Stem Cell Agency Speaks Out on Raelians and New Types of Human Beings
The California stem cell agency this morning is asking us all whether we remember the Raelians?
The agency, however, is not offering a $64,000 prize for the answer. No quiz show contest at the Oakland headquarters of the California Institute for Regenerative Medicine (CIRM), as the agency is formally known.
Instead the Raelian recollection is the lead-in to a cautionary note about the reports out of China that a researcher, who once worked at Stanford, has genetically altered two babies in embryo. The news has triggered an international flap about the ethics involved along with warnings about gene editing that results in "new kinds of human beings."
Writing on the agency's blog, Kevin McCormack, senior director for CIRM communications, said,
He noted the use of the CRISPR gene editing technique which has made it much easier to dip into the genetic process. McCormack wrote,
McCormack concluded,
The agency, however, is not offering a $64,000 prize for the answer. No quiz show contest at the Oakland headquarters of the California Institute for Regenerative Medicine (CIRM), as the agency is formally known.
Instead the Raelian recollection is the lead-in to a cautionary note about the reports out of China that a researcher, who once worked at Stanford, has genetically altered two babies in embryo. The news has triggered an international flap about the ethics involved along with warnings about gene editing that results in "new kinds of human beings."
Writing on the agency's blog, Kevin McCormack, senior director for CIRM communications, said,
"Remember the Raelians? Probably not. But way back in 2002 the group, some described them as a cult, claimed it had created the world’s first cloned baby. The news made headlines all around the world raising fears we were stepping into uncharted scientific territory. Several weeks later the scientist brought in by the Raelians to verify their claims called it an 'elaborate hoax.'"McCormack cautioned that ultimately the news out of China could amount to the same sort of thing.
He noted the use of the CRISPR gene editing technique which has made it much easier to dip into the genetic process. McCormack wrote,
"CRISPR has been making headlines all of its own in the last few years as a fast, cheap and efficient way of editing genes. CIRM supports research using CRISPR for problems such as sickle cell disease. The difference being that our research works with adults so any changes in their genes are just for them. Those changes are not passed on to future generations.
"The work making headlines around the world used CRISPR on embryos, meaning a child born from one of those embryos would pass those changes on to future generations. In effect, creating a new kind of human being."McCormack picked up a sample of reaction around the world, including a comment from Stanford bioethicist Hank Greely on CNBC. Greeley said that that if the report is accurate, the research is "criminally reckless, and I unequivocally condemn the experiment.”
McCormack concluded,
"Our best hope right now is that this is just a repeat of the Raelians. Our worst fear, is that it’s not."
Monday, November 26, 2018
Genetically Altered Babies: A Bit of a California Connection
This You Tube video produced by He Jiankui had drawn 19,723 views at the time of this posting along with nearly 300 comments, pro and con. The number of views jumped about 5,000 during one hour this morning.
The startling news about what is being described as the world's first gene-edited baby has a something of a California tie.
The scientist behind the work, He Jiankui, worked from 2010 to 2012 in the lab of Stephen Quake at Stanford University, who is participating in a $40 million genomics program backed by California's $3 billion stem cell agency.
A statement on the web site of Direct Genomics, a company He Jiankui founded, said,
In the stem cell agency's genomics project, Quake is the lead on a project dealing with cell differentiation.
California's Center for Genetics and Society in Berkeley released a statement on the news about the gene editing. Executive Director Marcy Darnovsky, said,
The startling news about what is being described as the world's first gene-edited baby has a something of a California tie.
The scientist behind the work, He Jiankui, worked from 2010 to 2012 in the lab of Stephen Quake at Stanford University, who is participating in a $40 million genomics program backed by California's $3 billion stem cell agency.
Like many other scientists, He Jiankui was trained in the United States, receiving his Ph.D. from Rice University.
A statement on the web site of Direct Genomics, a company He Jiankui founded, said,
"He was working on genome sequencing research during his postdoc training in the lab of Stephen Quake at Department of Bioengineering, Stanford University. Dr. He has multidisciplinary research background, such as in physical theory of network evolution, influenza virus, immune repertoire sequencing, single cell genomics and bioinformatics."Quake's lab is currently dealing with ultra high throughput DNA sequencing. On the lab's web site, a mission statement by Quake said,
"My work in single molecule biophysics led to the first demonstration of single molecule sequencing, and my research in this field has led me to become deeply involved in human genetics, immunology, and the development of new clinical diagnostics."Quake has not yet responded to a query concerning He Jiankui's work at Stanford.
In the stem cell agency's genomics project, Quake is the lead on a project dealing with cell differentiation.
California's Center for Genetics and Society in Berkeley released a statement on the news about the gene editing. Executive Director Marcy Darnovsky, said,
“If true, this amounts to unethical and reckless experimentation on human beings, and a grave abuse of human rights. We wish the best for the health of these babies, but strongly condemn the stunt that threatens their safety, and puts the rest of us at risk. Throwing open the door to a society of genetic haves and have-nots undermines our chances for a fair and just future.”
Thursday, November 15, 2018
California Kicks Off Major, New Foray into Gene Therapy: First by Any State in the Country
California today became the first state in the nation to launch itself into the sizzling field of gene therapy, backed by tens of millions of dollars and with the hope of creating treatments that could permanently cure afflictions ranging from hemophilia to cancer.
The move came today as the board of the $3 billion California stem cell agency opened its doors to funding gene therapy research that has reached the most advanced stage, clinical trials. The agency said that gene therapy -- minus stem cells -- is "valuable and worthy of pursuit."
"This is where the science is going," said Jeff Sheehy, chairman of the CIRM board's Science Subcommittee, at hearing earlier this month,
The state stem cell program has allocated $143 million for research programs next year that could include gene therapy.
A document prepared by the leadership of the agency, formally known as the California Insitute for Regenerative Medicine (CIRM), said,
In its action today, the stem cell agency did not announce any specific research awards. Rather it created a procedure for declaring that a gene therapy project with a regenerative element is a "vital research opportunity." That would allow CIRM to fund such a project under the terms of the ballot initiative that created the agency in 2004. Today's action is the first time that the agency has acted to use the "vital opportunity" provision.
CIRM has budgeted $143 million for next year in two areas where the new gene therapy initiative could come into play, clinical trials along with translational research that is intended to advance basic research into clinical stages.
The agency's new foray comes as its cash is running out. By the end of next year, it expects to have no more funding for new research. The agency is pinning its hopes of survival on a $200 million plus private fundraising effort this year and voter approval of a yet-to-be-written ballot initiative on the November 2020 ballot.
The gene therapy field is moving swiftly and could generate a result that would resonate with voters and help to win approval of an additional $5 billion for CIRM.
CIRM board members have acknowledged that its new gene therapy effort will mean more competition for the state's research dollars, including possibly less for stem cell research, which is the agency's fundamental reason for being.
The agency, however, has already awarded millions of dollars for gene therapy research that has a stem cell link, including a program at UCLA that has led to a successful treatment for what is known as the "bubble boy syndrome." However, that research has not yet moved into the marketplace.
The target of the UCLA treatment is an immune deficiency that is fatal and for which there is no successful treatment outside of the experimental trials that are still underway.
Bloomberg News has reported that Scott Gottlieb, head of the federal Food and Drug Administration(FDA), has described the field as "somewhat breathtaking." More than 500 experimental treatments are in the pipeline.
Gottlieb has said that he expects the FDA to approve 40 gene therapies by 2022 and possibly a cure for sickle cell anemia within 10 years. CIRM is deeply involved in a major national push on sickle cell supported by the National Institutes of Health.
The move came today as the board of the $3 billion California stem cell agency opened its doors to funding gene therapy research that has reached the most advanced stage, clinical trials. The agency said that gene therapy -- minus stem cells -- is "valuable and worthy of pursuit."
"This is where the science is going," said Jeff Sheehy, chairman of the CIRM board's Science Subcommittee, at hearing earlier this month,
The state stem cell program has allocated $143 million for research programs next year that could include gene therapy.
A document prepared by the leadership of the agency, formally known as the California Insitute for Regenerative Medicine (CIRM), said,
"For CIRM and the patients it aims to serve, it is vital to support technologies which prove to be highly complementary and augmenting to stem cells, such as gene therapy."Gene therapy treatments are expected to be quite expensive, with some forecasts running in the $1 million to $2 million range. Supporters of gene therapy argue that the cost is justified because gene therapy can be a total cure that would eliminate the need for also very expensive lifelong treatments of chronic diseases.
In its action today, the stem cell agency did not announce any specific research awards. Rather it created a procedure for declaring that a gene therapy project with a regenerative element is a "vital research opportunity." That would allow CIRM to fund such a project under the terms of the ballot initiative that created the agency in 2004. Today's action is the first time that the agency has acted to use the "vital opportunity" provision.
CIRM has budgeted $143 million for next year in two areas where the new gene therapy initiative could come into play, clinical trials along with translational research that is intended to advance basic research into clinical stages.
The agency's new foray comes as its cash is running out. By the end of next year, it expects to have no more funding for new research. The agency is pinning its hopes of survival on a $200 million plus private fundraising effort this year and voter approval of a yet-to-be-written ballot initiative on the November 2020 ballot.
The gene therapy field is moving swiftly and could generate a result that would resonate with voters and help to win approval of an additional $5 billion for CIRM.
CIRM board members have acknowledged that its new gene therapy effort will mean more competition for the state's research dollars, including possibly less for stem cell research, which is the agency's fundamental reason for being.
The agency, however, has already awarded millions of dollars for gene therapy research that has a stem cell link, including a program at UCLA that has led to a successful treatment for what is known as the "bubble boy syndrome." However, that research has not yet moved into the marketplace.
The target of the UCLA treatment is an immune deficiency that is fatal and for which there is no successful treatment outside of the experimental trials that are still underway.
Bloomberg News has reported that Scott Gottlieb, head of the federal Food and Drug Administration(FDA), has described the field as "somewhat breathtaking." More than 500 experimental treatments are in the pipeline.
Gottlieb has said that he expects the FDA to approve 40 gene therapies by 2022 and possibly a cure for sickle cell anemia within 10 years. CIRM is deeply involved in a major national push on sickle cell supported by the National Institutes of Health.
Labels:
bond election,
gene therapy,
grantmaking,
Prop. 71
Monday, November 12, 2018
Combating Stem Cell Snake Oil: A Primer From a California Researcher
Hundreds of dubious, unregulated stem cell clinics exist throughout the country with the most in California. Desperate people seeking help have been maltreated and fleeced. What to do?
 UC Davis stem cell research Paul Knoepfler has produced a useful guide to reporting dubious
UC Davis stem cell research Paul Knoepfler has produced a useful guide to reporting dubious
activities along with a list of state and federal agencies that could have a role.
In an item on his blog last week, Knoepfler wrote,
 UC Davis stem cell research Paul Knoepfler has produced a useful guide to reporting dubious
UC Davis stem cell research Paul Knoepfler has produced a useful guide to reporting dubious activities along with a list of state and federal agencies that could have a role.
In an item on his blog last week, Knoepfler wrote,
"I would emphasize concrete reasons for concern such as the use of an unapproved stem cell drug product by the clinic, a physician practicing outside their area of expertise so putting their patients at risk, false marketing, and potential or documented (if they’ve already happened) patient harms.Knoepfler continued,
"For some clinics that aren’t led by physicians, I would also emphasize the risks of non-physicians such as chiropractors or Ph.D.s doing procedures for which they aren’t trained or licensed. I think patients (or people communicating on their behalf) making complaints about clinics and their personnel will have the greatest impact."
"We can make a difference by pushing back on the worst clinics. Of course, not every action by those of us in the stem cell arena who are concerned about predatory clinics will hit a bullseye to make real change, but sometimes it has happened in the past and will happen again in the future too."I would add that the activities and stories that emerge from these dubious enterprises damage the reputation of the field as a whole. Indeed, when I talk to folks in the general public, the clinics' offerings are the most often mentioned reference they have.
As California's stem cell agency moves closer to seeking more billions from voters in 2020, fraudulent stem cell activity could create much confusion about the legitimacy of the entire field. Supporters might want to keep that in mind. But critics should as well. Fleecing the public with unproven therapies is unhealthy for those who are swindled but also for society in general.
Thursday, November 08, 2018
California's Stem Cell Agency Opening Door to Pumping More Millions into the Sizzling Gene Therapy Market
 |
| Gene therapy graphic from FDA |
The action came when a key panel of the agency's governing board this morning approved its first-ever procedure for awarding millions of dollars for research not connected to stem cells.
"This is where the (regenerative) science is going," said Jeff Sheehy, chair of the Science Subcommittee of the board of the California Institute for Regenerative Medicine (CIRM), as the Oakland-based agency is formally known.
Under Prop. 71, the ballot initiative that created the $3 billion agency in 2004, CIRM is limited to financing research involving stem cells in some fashion. However, a provision of the measure also allows the support of a "vital research oppportunity" under certain conditions. Today's action formalizes the process for making that finding.
CIRM said in in a document that it is "vital" that the agency expand its horizons.
"CIRM has supported projects that combine stem cell and gene therapy technologies, such as the gene-corrected stem cell transplants at UCLA that essentially cured 5-year old Evangelina Padilla Vaccaro and several CAR-T cell approaches using stem memory T cells that aim to tackle various cancers.
"The support of stem cell research that contributes to these treatments is and will continue to be the core of CIRM funding. However, treatment opportunities in regenerative medicine that utilize gene therapy technologies but not necessarily stem cells are also valuable and worthy of pursuit."
The agency's statement continued,
"The field of regenerative medicine brings together technologies that include stem cells, gene therapy, and tissue engineering that in many cases combine to produce a therapeutic product. In some cases, one technology leads the way. For CIRM and the patients it aims to serve, it is vital to support technologies which prove to be highly complementary and augmenting to stem cells, such as gene therapy."
Several directors noted during today's meeting that the change could lead to less cash for purely stem cell projects.
The field of gene therapy has attracted widespread interest within the regenerative medicine industry and among federal medical regulators. Scott Gottlieb, commissioner of the Food and Drug Administration, earlier this year announced steps to speed development of the therapies.
The field of gene therapy has attracted widespread interest within the regenerative medicine industry and among federal medical regulators. Scott Gottlieb, commissioner of the Food and Drug Administration, earlier this year announced steps to speed development of the therapies.
"'The pace of progress in gene therapy has been somewhat breathtaking,' he (Gottlieb) said, with more than 500 experimental drugs now in development. 'The promise is becoming very much a reality.'"
In April, Novartis AG ponied up $8.7 billion to buy AveXis Inc., of Illinois to strength its efforts to produce a marketable gene therapy.
Gottlieb has said that he expects the FDA to approve 40 gene therapies by 2022 and possibly a cure for sickle cell anemia within 10 years.
CIRM's new gene therapy policy will go before the full CIRM board one week from today where it is expected to be approved. Additional information on the policy can be found on the Science Subcommittee agenda.
Tuesday, November 06, 2018
An FDA High Sign for a California Stem Cell Agency Bet: San Diego Biotech Business Earns FDA Speed-Up Approval
A San Diego firm backed by nearly $24 million from the California stem cell agency scored this week with a special designation from the federal government that could help speed approval of its therapy for a type of blood cancer.
The firm is Poseida Therapeutics, Inc., which is testing the safety of a therapy for a type of blood cancer in a phase one clinical trial.
The firm announced yesterday that the Food and Drug Administration (FDA) had granted Regenerative Medicine Advanced Therapy (RMAT) status for the adult stem cell-connected treatment. The designation is intended to expedite development of the multiple myeloma therapy.
Eric Ostertag, CEO of the firm, said in a news release, that Poseida's potential treatment, P-BCMA-101, "is the first anti-BCMA CAR-T therapy to receive RMAT designation from the FDA and underscores the urgent need for new treatment options for multiple myeloma."
He continued,
The stem cell agency, known formally as the California Institute for Regenerative Medicine (CIRM), has invested in 49 clinical trials. Five have garnered RMAT designation, which can significantly speed development of a commercial product. As of September, the FDA had granted only 24 RMAT designations nationally.
Last Sepetember, Geoff Lomax, CIRM senior officer for medical affairs and strategic centers, wrote in cell&gene that the two-year-old, RMAT program streamlines therapy development by enabling possible priority FDA review and accelerated federal approval.
Longitude Capital of Menlo Park, Ca., is a major investor in the firm, pumping in tens of millions of dollars.
The firm is Poseida Therapeutics, Inc., which is testing the safety of a therapy for a type of blood cancer in a phase one clinical trial.
The firm announced yesterday that the Food and Drug Administration (FDA) had granted Regenerative Medicine Advanced Therapy (RMAT) status for the adult stem cell-connected treatment. The designation is intended to expedite development of the multiple myeloma therapy.
Eric Ostertag, CEO of the firm, said in a news release, that Poseida's potential treatment, P-BCMA-101, "is the first anti-BCMA CAR-T therapy to receive RMAT designation from the FDA and underscores the urgent need for new treatment options for multiple myeloma."
He continued,
“Initial Phase 1 data presented at the CAR-TCR Summit earlier this year included encouraging response rates and safety data, including meaningful responses in a heavily pretreated population...."Ostertag said the firm expects to have more data by the end of the year.
The stem cell agency, known formally as the California Institute for Regenerative Medicine (CIRM), has invested in 49 clinical trials. Five have garnered RMAT designation, which can significantly speed development of a commercial product. As of September, the FDA had granted only 24 RMAT designations nationally.
Last Sepetember, Geoff Lomax, CIRM senior officer for medical affairs and strategic centers, wrote in cell&gene that the two-year-old, RMAT program streamlines therapy development by enabling possible priority FDA review and accelerated federal approval.
Longitude Capital of Menlo Park, Ca., is a major investor in the firm, pumping in tens of millions of dollars.
Labels:
clinical trials,
poseida,
rmat,
venture capital
Thursday, November 01, 2018
California Stem Cell Agency Moving to Expand its Reach into Big Market for Gene Therapy
 |
| The definition of gene therapy under proposed changes for research funding by the California stem cell agency. CIRM chart |
The change comes as the stem cell agency is looking to generate results that are likely to resonate with voters in November 2020 who may be asked to provide an additional $5 billion in funding for the program. The agency expects to run out of cash by the end of next year.
Gene therapy has received considerable attention in the last few years. Yesterday, Orchard
Therapeutics, a British gene therapy firm that has links to CIRM (also see here) and research by Donald Kohn of UCLA, raised $200 million in an initial stock offering. The company said in its prospectus that the total market potential "in the diseases areas underlying our five lead programs could be greater than $2 billion annually."
 |
| Donald Kohn, UCLA photo |
The Science Subcommittee of the agency is expected to approve extension of its gene therapy efforts next Wednesday in a teleconference meeting that will be available globally through the Internet.
Under the provisions of the ballot measure that created the agency in 2004, the agency is limited in scope. But exceptions are possible if a finding is made that a "vital research opportunity" exists.
Next week's meeting is expected to formalize the process of making that determination in regards to gene therapy that does not involve stem cells. It will require a 2/3 vote of CIRM's grant review group, among other things.
Members of the public can participate in the hearing remotely via the Internet or at locations in Oakland, San Francisco, La Jolla, Riverside and Napa. Directions can be found on the agenda.
Labels:
bond measufre,
gene therapy,
grant making,
Prop. 71
Subscribe to:
Comments (Atom)

