"Stem cell crusader sparks new hope....Regenerative medicine could be a $120 billion industry by 2030"
But only three days later, that article was overwhelmed by a wave of stories with a much different flavor. They carried reports of unproven therapies, possibilities of fraud, lack of regulation and fearful, expensive consequences for desperate patients.
That theme continued even as recently as this past weekend as The Economist carried a piece headlined,
"A dish called hope: The flourishing, unregulated industry in expensive, experimental treatments"So which is it? Hope for legitimate cures and oodles of cash for stem cell companies? Or "hope" for treatments that do not work and have sometimes damaged both the bodies and wallets of the patients?
The situation is more than a minor public relations headache for the supporters of stem cell research, be they patients, stem cell companies or the $3 billion California stem cell agency, which is on a campaign to ease federal regulation of stem cell clinical trials.
For all practical purposes, the public generally does not make much of distinction between the legitimate research conducted by institutions such as Stanford and the so-called "miracles" reported about the late hockey great Gordie Howe and others. It all goes into the same cognitive bag. The stories about mysterious and fearsome tumors found in little-known patients attract little widespread attention.
But UC Davis scientist Paul Knoepfler and bioethicist Leigh Turner of the University of Minnesota ripped open that bag with their study last month that reported for the first time that nearly 600 dubious stem cell clinics are peddling their unproven therapies across the country. Their report supported the view that the stem cell field is rife with snake oil merchants. For the general public, which does not delve deeply into medical research, the perspective could well be described as, "You seen one stem cell therapy, you seen 'em all."
Does this mean that these clinics should be ignored and shoved quietly off into a corner in order to avoid besmirching legitimate efforts? Of course not. It certainly appears that the FDA and other state regulatory bodies can do a better job. But stiffer regulations are not going to come anytime soon, despite an FDA hearing on the topic in September.
However, the situation DOES mean that folks like Knoepfler and Turner should continue to speak out along with many other scientists who do not want their efforts blackened by the snake oil men. Researchers can work with their institutions' PR departments to place op-ed pieces, find speaking engagements and gin up TV and radio interviews. Blogs, like the one produced by Knoepfler, can be started. The International Society for Stem Cell Research should revive its public education efforts to help patients and the general public understand the facts about stem cell research. It should also reinvigorate its warnings about dubious therapies, which were throttled back a few years ago, reportedly after legal threats were made.
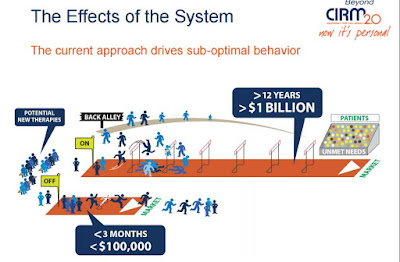
California's stem cell agency has a special concern. It is trying to "de-risk" development of therapies by a variety of means and lure biotech and Big Pharma into the stem cell game in a bigger way. This summer the agency is offering $75 million to entice a private partner into the state's first-ever, public-private partnership to create a stem cell cure.
The private sector has shied away from the stem cell business, as the stem cell agency's CEO, Randy Mills, has remarked on multiple occasions. One of the reasons involves the public climate and perception of the field. It is hard for companies to invest hundreds of millions of dollars or billions when the stem cell field is burdened with public perception and regulatory obstacles.
Selling the stem cell story in a realistic way is not necessarily an easy task. Nuances must be respected, but excitement is also needed. Balancing it all is a challenge for the men and women in the trenches. But without a good push, development of therapies will be slower, and, as Mills has noted, people will undoubtedly die who likely would have benefited from a more timely treatment.